Calithera is working registered scientific trials on its goods to analyze their protection, regardless of whether they are effective in patients with certain gene mutations, and how nicely they perform in blend with other therapies. The organization have to accumulate thorough facts on hundreds of people. While some of its trials are in early phases and require only a compact variety of clients, other folks span much more than 100 study facilities throughout the world.
“In the lifetime-sciences world, just one of the major difficulties we have is the great amount of knowledge we generate, additional than any other organization,” suggests Behrooz Najafi, Calithera’s lead facts engineering strategist. (Najafi is also main data and technological innovation officer for wellbeing-treatment tech organization Innovio.) Calithera should retail outlet and control the facts even though creating positive it’s easily offered when essential, even a long time from now. It also have to comply with particular Fda needs on how the data is generated, saved, and utilized.
Even one thing seemingly as uncomplicated as upgrading a file server must abide by a strictly outlined Food and drug administration protocol with numerous testing and assessment steps. Najafi states all this compliance-relevant facts wrangling can increase 30% to 40% to the overhead of a firm like his, in equally immediate expense and hours of staff members time. These are methods that could normally be set towards more investigation or other worth-additional things to do.
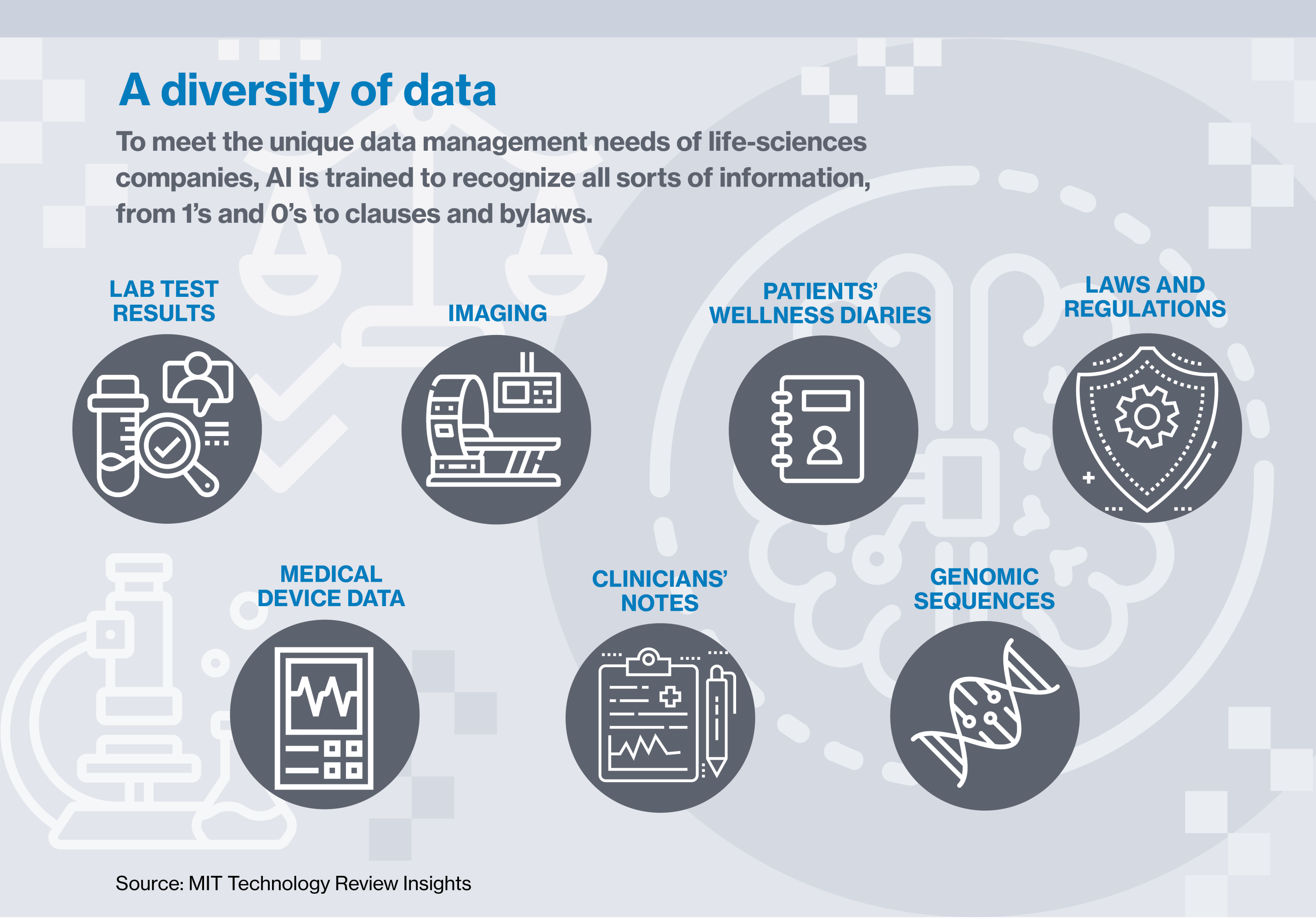
Calithera has sidestepped much of that additional price and vastly enhanced its skill to observe its knowledge by putting it in what Najafi calls a safe “storage container,” a safeguarded place for controlled content material, element of a greater cloud document management application, largely pushed by synthetic intelligence. AI in no way sleeps, under no circumstances receives bored, and can find out to distinguish among the hundreds of distinct varieties of paperwork and kinds of facts.
Here’s how it functions: clinical or client knowledge is place into the program and scanned by AI, which recognizes unique capabilities that pertain to accuracy, completeness, compliance with polices, and other elements of the facts. AI can flag when there’s a missing test result, or when a affected person has not submitted a required diary entry. It appreciates who’s allowed to access specified sorts of facts and what they are and are not permitted to do with it. It can detect ransomware attacks and head them off. And it can automatically doc all that to the satisfaction of the Food and drug administration or any other regulatory entire body.
“This tactic usually takes the compliance load off of us,” Najafi claims. After knowledge from its several exploration websites is in the system, Calithera knows that the AI will make confident it is risk-free, total, and compliant with all laws, and will flag any issues.
Controlling drug discovery details to comply with the desires of study and the requirements of regulators can be, as Najafi observes, onerous and high priced. The lifestyle-sciences industry can borrow information management techniques and platforms produced for other industries, but they ought to be modified to deal with the levels of security and validation, and the comprehensive audit trails, that are a way of lifetime for drug builders. AI can streamline these responsibilities, enhancing the protection, consistency, and validity of data—freeing up overhead for drug firms and investigate businesses to apply to their core mission.

An intricate knowledge administration environment
Regulatory compliance helps make sure that new drugs and equipment are protected and get the job done as meant. It also shields the privacy and personal data of the countless numbers of patients who participate in clinical trials and article-marketplace investigation. No issue their size—enormous world-wide conglomerates or tiny startups trying to get a single item to market—drug developers should adhere to the exact same standard tactics to doc, audit, validate, and safeguard each individual shred of info linked with a scientific demo.
When researchers run a double-blind examine, the gold standard for proving the efficacy of a drug, they have to hold patients’ data nameless. But they must simply de-anonymize the info later, producing it identifiable, so clients in the handle team can obtain the exam drug, and so the business can track—sometimes for years— how the solution performs in serious-planet use.
The details management burden falls challenging on rising and midsize biosciences businesses, claims Ramin Farassat, chief method and item officer at Egnyte, a Silicon Valley program business that can make and supports the AI-enabled info administration platform employed by Calithera and quite a few hundred other existence-sciences companies.
“This technique usually takes the compliance load off of us,” Najafi says. When details from its quite a few study websites is in the system, Calithera knows that the AI will make absolutely sure it is safe and sound, entire, and compliant with all regulations, and will flag any complications.
Obtain the total report.
This articles was made by Insights, the custom information arm of MIT Know-how Overview. It was not published by MIT Technological innovation Review’s editorial personnel.






More Stories
Evolution of a Vital Software – The Internet Browser
How Not to Write Your Curriculum Vitae
Importance of Information and Communications Technology (ICT) In Our Daily Life